BiC BRIDGE
Biomarkers, or markers providing information on the health status of a person, measure cellular, biochemical or molecular changes in human tissues as well as cells or fluids. They contribute to future diagnostics and treatment. The development of biomarkers is time consuming and expensive, requiring the involvement of industry from early stages to better direct the research. Researchers lack the knowledge of complex commercialization processes and small and medium-sized enterprises (SMEs) have poor understanding of regulatory frameworks.
BIC BRIDGE built on the tools developed by the project BIC.
Budgets
in numbers
-
0.76MillionTotal
-
0.58MillionErdf
-
0.00MillionEni + Russia
-
0.00MillionNorway
Achievements
While working directly with the users (SMEs essentially), BiC BRIDGE added two steps to the tool that was already developed within the main project. These steps correspond to the last phases before commercialisation. In practice, this means guidelines to be followed by SMEs when they plan to launch a new test on the market. The commercial goal is thus central in these phases, while the technical and regulatory processes are crucial supportive steps.
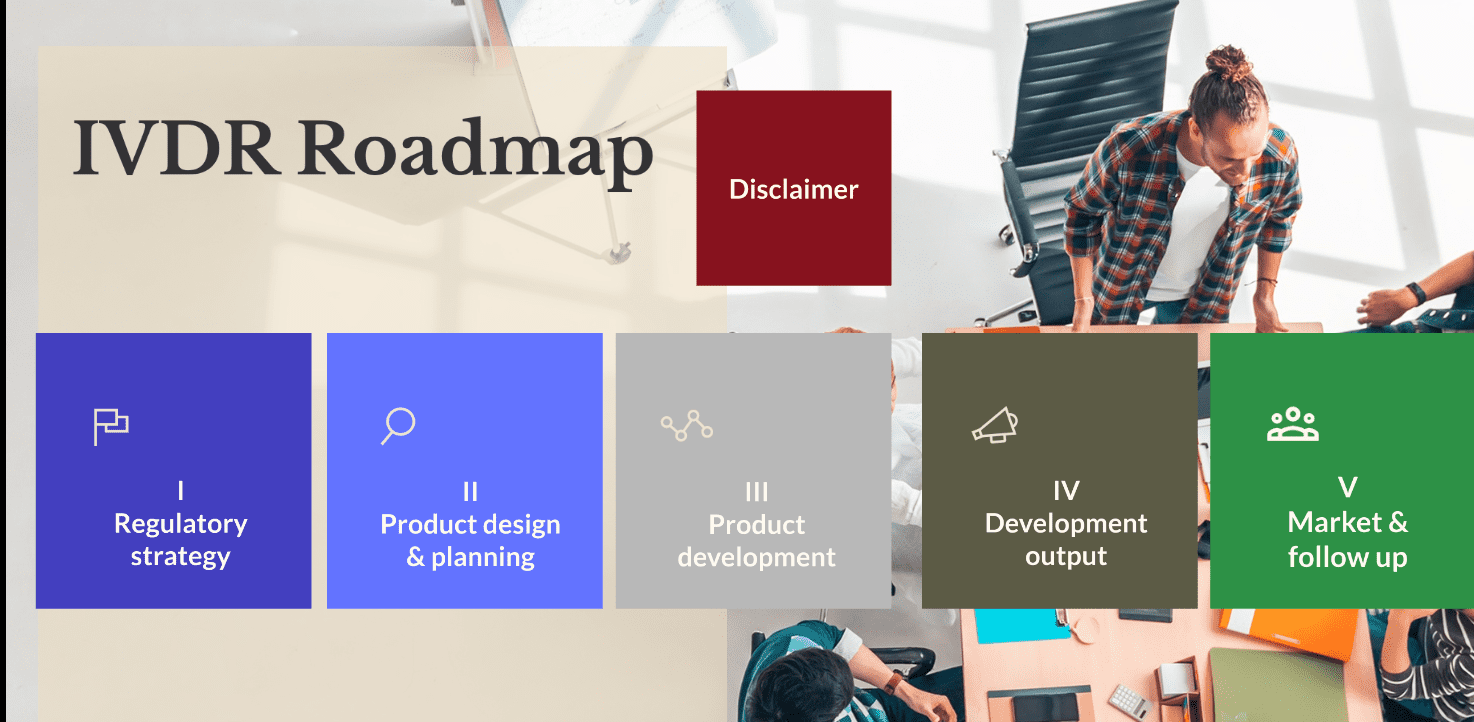
BiC BRIDGE has also developed an IVDR (In Vitro Diagnostics Regulatory) roadmap, which allows users to visualize in a dynamic way the regulatory process. It provides a live understanding of the interaction between the technical, commercial and regulatory processes. Finally, the project created an educational toolbox, including several tutorials, access to all recorded webinars, as well as a visual explanation of how to use the BiC tools. The project made sure to shorten the time between the discovery of a biomarker and its market entry also for the future. The long process of patenting, licensing and commercialising a biomarker discovery is now easier and faster.
Outputs
In Vitro Diagnostics Regulatory Roadmap
Biomarker Commercialization Guide
Project Stories
-
01.12.2020
Commercialising biomarkers faster to improve medical treatment
A project was set into motion in 2017 with the ambitious aim to facilitate and standardize the commercialization process of new biomarkers. Biomarkers are increasingly important in disease risk prediction, diagnosis, prognosis and treatment response. The BIC project concluded in September 2020 and brought about some tools that can help medical companies to bring new biomarkers to the market faster.Read full story -
25.04.2022
Enabling the market uptake of biomarkers used in diagnostics and treatment
In the Interreg project BIC, partners from six countries around the Baltic Sea worked together to bring biomarkers into medical companies’ offers faster. In this way, BIC pushed forward personalised diagnostic and treatment for patients across the region.Read full story
Partners
Ideklinikken, Aalborg University Hospital, The North Denmark Region
- TownAalborg
- RegionNordjylland
- CountryDenmark
- RepresentativeValérie Daussin-Laurent
- Phone
- E-Mail
- Web
Wroclaw Technology Park
- TownWrocław
- RegionMiasto Wrocław
- CountryPoland
- RepresentativeJoanna Kułdo
- Phone
- E-Mail
- Web
BioCon Valley® GmbH
- TownGreifswald
- RegionVorpommern-Greifswald
- CountryGermany
- RepresentativeThomas Karopka
- Phone
- E-Mail
- Web
Tartu BT Park OÜ
- TownTartu
- RegionLõuna-Eesti
- CountryEstonia
- RepresentativeEleri Seer
- Phone
- E-Mail
- Web
University of Turku
- TownTurku
- RegionVarsinais-Suomi
- CountryFinland
- RepresentativePauli Ollikka
- Phone
- E-Mail
- Web
Turku Science Park Ltd
- TownTurku
- RegionVarsinais-Suomi
- CountryFinland
- RepresentativeTero Piispanen
- Phone
- E-Mail
- Web
Labmaster Ltd
- TownTURKU
- RegionVarsinais-Suomi
- CountryFinland
- RepresentativeKonstantin Denessiouk
- Phone
- E-Mail
- Web
Dianox APS
- TownAlbertslund
- RegionKøbenhavns omegn
- CountryDenmark
- RepresentativeHuram Konjen
- Phone
- E-Mail
- Web
EATRIS
- TownAmsterdam
- RegionGroot-Amsterdam
- CountryNetherlands
- RepresentativeFlorence Bietrix
- Phone
- E-Mail
- Web
-
Project managerValerie Daussin LaurentAalborg University Hospital
-
Legal representativeJane Bjerregaard RasmussenIdeklinikken, Aalborg University Hospital, The North Denmark Region
-
Financial managerGitte WimmerRegion Nordjylland
-
Communication managerMerja TieahoTurku Science Park Oy